Company Description
We are SGS - world's leading testing, inspection and certification company. We are recognized as the global benchmark for sustainability, quality and integrity. Our 96,000 employees operate a network of 2,600 offices and laboratories around the world. In Finland SGS employs 250 experts. Our headquarters is located in Helsinki, but we also have offices in Espoo, Tuusula, Kotka, Karkkila, Tampere and Rauma. More information on our services: https://www.sgs.com/en-fi.
Job Description
SGS Fimko Ltd has been designated as a notified body according to the new medical device regulation (EU) 2017/745 as one of the few organizations in Europe.
As a clinical expert in the team, your main duties include collaborating with our lead auditors to evaluate the clinical evidence of products according to the regulation. This includes reviewing documentation, performing assessments and writing reports. You will also be in charge of coordinating the work for our external clinical experts. You will take part in developing the Notified Body team and training our personnel and customers.
We offer a permanent hourly contract with monthly hours generally between 20-60 hours. Work hours and times can be discussed.
Qualifications
This position requires a medical degree and at least 5 years of work experience including direct work experience in patient care and research experience, either in conducting preclinical or clinical trials or assessing clinical data.
The selected clinical expert will focus on active medical devices, but experience with IVD devices is also appreciated. A background or experience in health technology assessment (HTA) is regarded a merit for the applicant.
Good communication and team-working skills are needed, as well as an analytical approach. The position requires fluent English skills. Ability to understand Finnish is helpful in everyday activities. Knowledge of the quality system standard EN ISO 13485, medical device standards and industry legislation is appreciated.
Additional Information
SGS offers stability, opportunities for growth and the chance to make a difference. SGS Fimko is the SGS group's most significant center of expertise in medical technology. With us, you get to work in an international environment with the latest technology and interesting challenges. We also test medical devices in Finland, so you have the best experts of the field at your side. We offer you a comprehensive onboarding to your role.
If you have any questions, please contact NB Manager Seppo Vahasalo p. +358 40 5609 500. Send us your CV and application by 31st July 2024.
#Bethechange #BeSGS
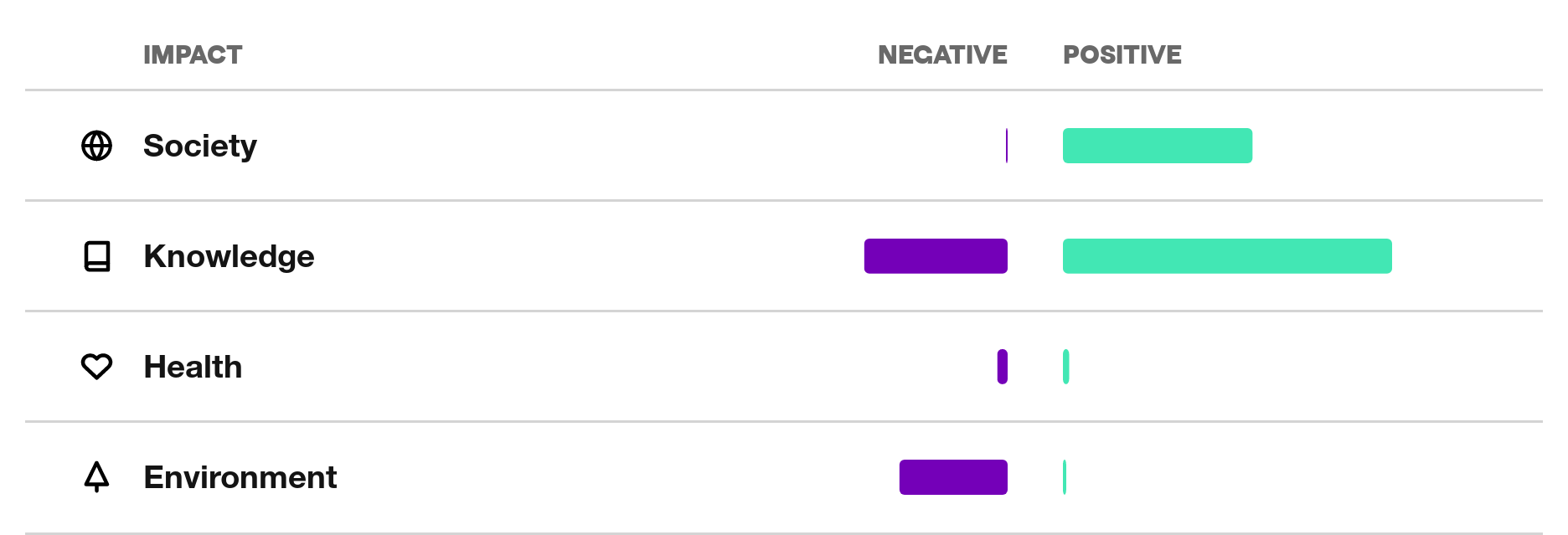
SGS Fimko creates its most significant negative impacts or uses resources in scarce human capital and waste, in categories Knowledge and Environment.
What is impact profile?
The net impact profile was modeled by the Upright Project. The profile is based on scientific research's understanding of the effects of various products and services. The profile tells you which things you are involved in influencing through this job. You can find more information about Upright here.