We are now looking for experienced Quality management professional to be the core player of our SAP Center of Excellence. You will be leading the solution side E2E process development together with business representatives and SAP CoE colleagues. The ideal candidate will bring extensive knowledge of quality systems and pharmaceutical regulations to guide and improve quality processes across the organization.
SAP CoE (Center of Excellence) is a midsize team responsible for SAP Platform activities, developments, and business support. We work together with an extensive partner network to enable Orion's core business processes to be developed with the latest technologies.
The role is permanent and located in Espoo or Turku offices in Finland, and our team follows Orion's hybrid mode of working.
Description of position
In this role you will analyze and understand existing business processes and develop them for future business needs by considering specific requirements and best practices. The QM module is comprehensive, encompassing aspects such as the inspection process, environmental monitoring during manufacturing, managing laboratory materials and managing quality cases.
Your main responsibilities include:
- Develop, implement with partners, and continuously improve Quality management (QM) module and its E2E flows in compliance with regulatory standards;
- Provide guidance on quality related regulatory changes to other IT stakeholders;
- Proactively seeking opportunities to apply new innovative solutions and realize the value of the existing assets, both in close collaboration with the internal and external stakeholders and partners;
- Managing the IT development backlog for the Quality management domain, in collaboration and alignment with the business stakeholders and broader IT organization;
- Leading and contributing to project and enhancement work of all sizes within your own process area;
- Taking ownership of the end-to-end lifecycle of IT solutions and platforms in your area and ensuring business fit, service levels, business continuity, solution documentation and quality standards are met.
The job description is meant to be a guideline as the position is new in the SAP CoE. Job role will be adjusted with the candidate's background and own interest areas.
Description of unit
Orion Information Management (IM) employs +80 people, of which approximately two thirds are in Espoo. Other locations are Turku, Kuopio, Hanko, Mumbai, and Nottingham. We support Orion's business in achieving its strategic targets not only by ensuring the availability of IT services but also by constantly looking for new innovations to match the current and future business needs.
We offer
Orion's Information Management works in close collaboration with the business functions to solve interesting problems. As a member of this community, you can contribute and affect how Orion improves our customers' well-being. We have a good working atmosphere based on common values and mutual trust. Please visit our website to find further information about Orion as an employer: https://www.orion.fi/en/careers/orion-as-an-employer/
Requirements
Skills and competences needed for this role:
- Suitable university degree and/or other relevant working experience in SAP platform area
- In-depth understanding of business processes and their mapping in SAP in respective quality area
- Experience in SAP QM module
- Experience of pharma industry and its Good Manufacturing Practices (GMP) is seen as an asset
- Knowing SAP S/4 Hana ecosystem is beneficial
- Fluent communication in English
- Good networking skills and energetic working approach
- Ability and willingness to learn and grow in the role, and potentially beyond
Additional Information
We look forward to hearing from you as soon as possible. We will start conducting interviews during the application period, and the last day to apply is 5th of January 2025.
For additional details, please contact Heli Uusitalo, Head of SAP CoE via LinkedIn or heli.uusitalo@orion.fi.
#LI-ORION
Approved medical examination which also includes drug testing is required prior to the employment. We will also carry out a security clearance prior to the employment for the selected person.
Orion Corporation operates in more than 30 countries, where we Orionees, 3600 in total, work in all kinds of positions. Among us there are Research Scientists, Laboratory Technicians, Engineers and IT Specialists as well as people working in Production, just to mention some examples. We offer diverse and responsible jobs to our personnel in a truly multi-disciplinary work environment. We encourage people to develop their competences and offer opportunities to affect the job description and creating their own career path at Orion. We are searching for top talents who are ready to share our passion for the work that we do.
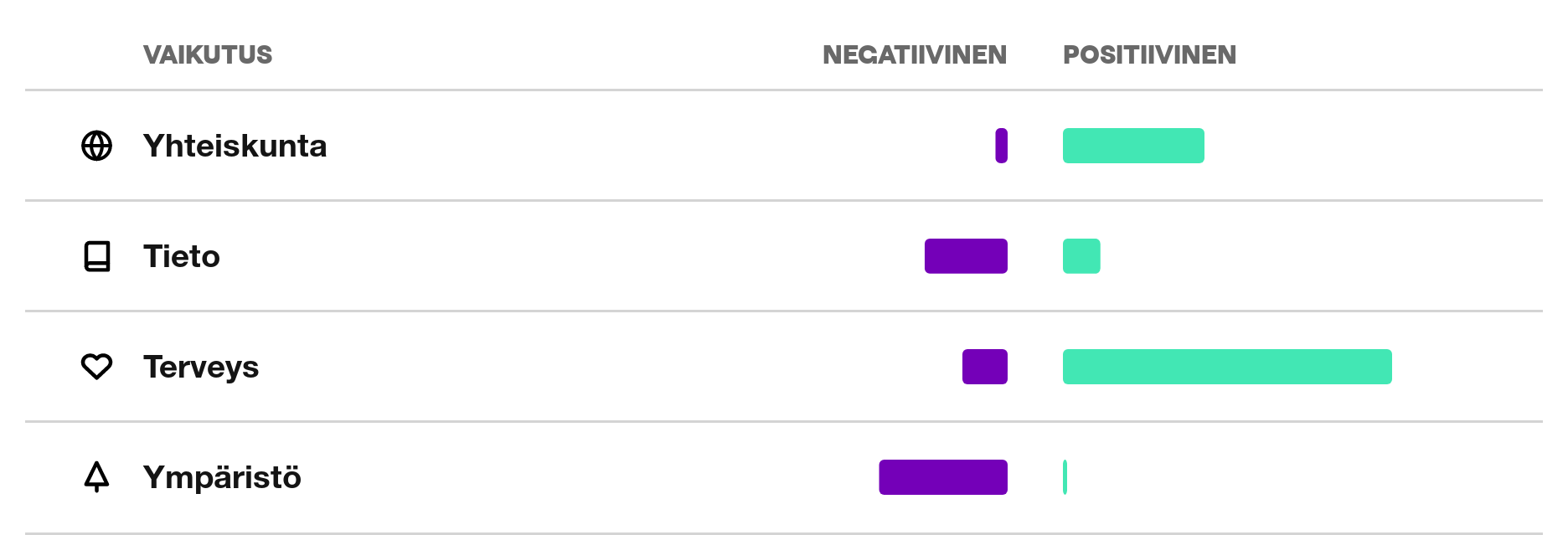
Orion synnyttää merkittävimmät negatiiviset vaikutuksensa tai käyttää resursseja liittyen kasvihuonekaasupäästöihin ja niukkaan osaamispääomaan, luokissa Ympäristö ja Tieto.
Mikä vaikuttavuusprofiili?
Nettovaikutusprofiilin on mallintanut Upright Project. Profiili perustuu tieteellisen tutkimuksen ymmärrykseen erilaisten tuotteiden ja palveluiden vaikutuksista. Profiili kertoo mihin asioihin sinäkin olet mukana vaikuttamassa tämän työpaikan kautta. Lisätietoa Uprightista löydät täältä.